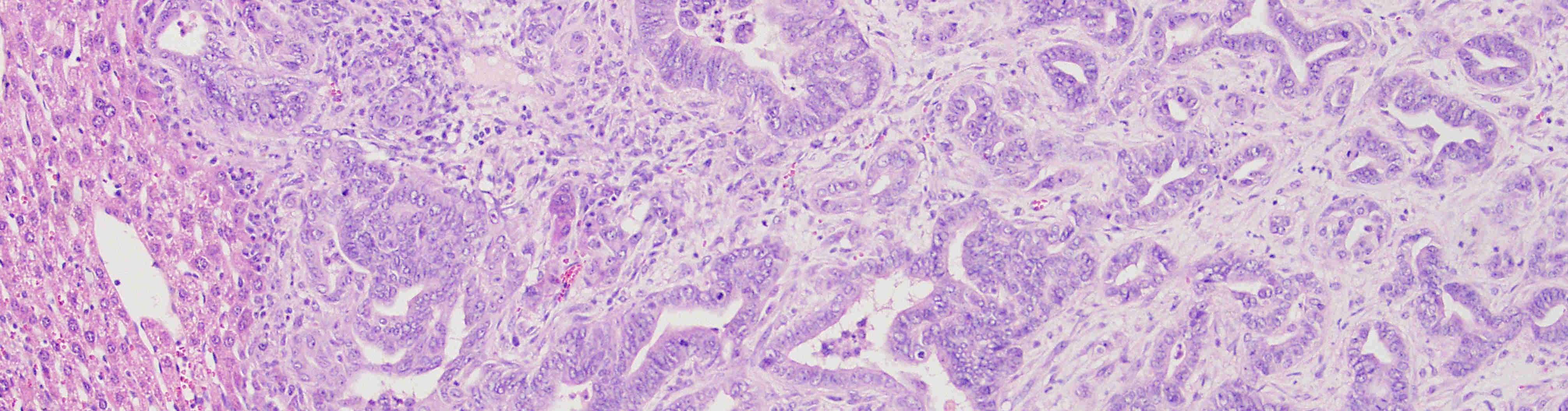
Cancer metastasis is the major cause of death in the majority of solid cancers. Therefore our aim is to understand the mechanisms that control cancer progression and metastasis in order to identify novel implications for the clinics. Metastasis is a multistep process, with a complex evolution, where cells leave the primary tumor and seed to distant organs. Every step during metastasis adds considerable heterogeneity to the tumor and includes the acquisition of stem cell features. These cancer stem cell populations have distinct molecular, genetic and phenotypic features, which jointly increase the risk of therapy resistance. However, even if cells show similar intrinsic features, the tumour microenvironment can control phenotypic plasticity of cancer stem cells.
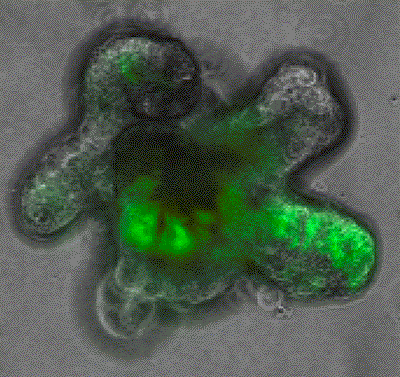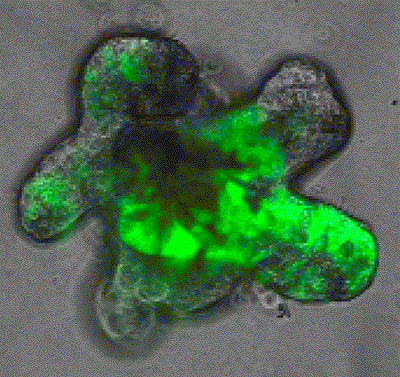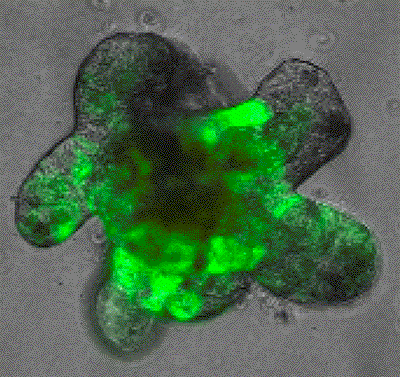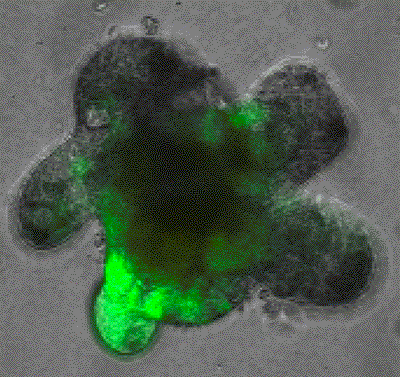
We utilise unbiased multi-omics (transcriptome, epigenome, methylome) at single cell level, functional genomics and lineage tracing approaches to analyse stem cell plasticity with complex in vivo models of metastatic colorectal cancer. These genetically engineered mouse models contain alterations in patient relevant genes and generate tumors with high similarity in progression, histology and molecular characteristics to human metastatic colorectal cancer. Further, these models allow tumor progression in the native tumour microenvironment, which enables us to investigate the role of non-cell-autonomous factors that control cancer stem cells features during metastasis. Our lab has a strong expertise in the use of genetically engineered mouse models, to analyse each step of molecular and cellular changes during the metastatic process longitudinally and in response to therapy. We also use primary clinical samples and patient derived organoids to better understand the most lethal stages of the disease.
To achieve this our research focuses on the following major topics:
- Functional characterisation of cancer stem cells in tumor-evolution
- Molecular and cellular basis of cancer stem cell niches
- Analysis of cancer stem cells and the interaction with the tumour microenvironment as driver of therapy resistance
Our research will broaden our understanding of the mechanisms that control metastatic progression and will help to find therapeutic vulnerabilities of late stage cancers.