Major Research Topics in HI-STEM are:
- Hematopoietic Stem Cells and Leukemia
- Solid Tumors, Metastasis and Therapy Resistance
- Stem Cells and Tumor Microenvironment
I. Hematopoietic Stem Cells and Leukemia
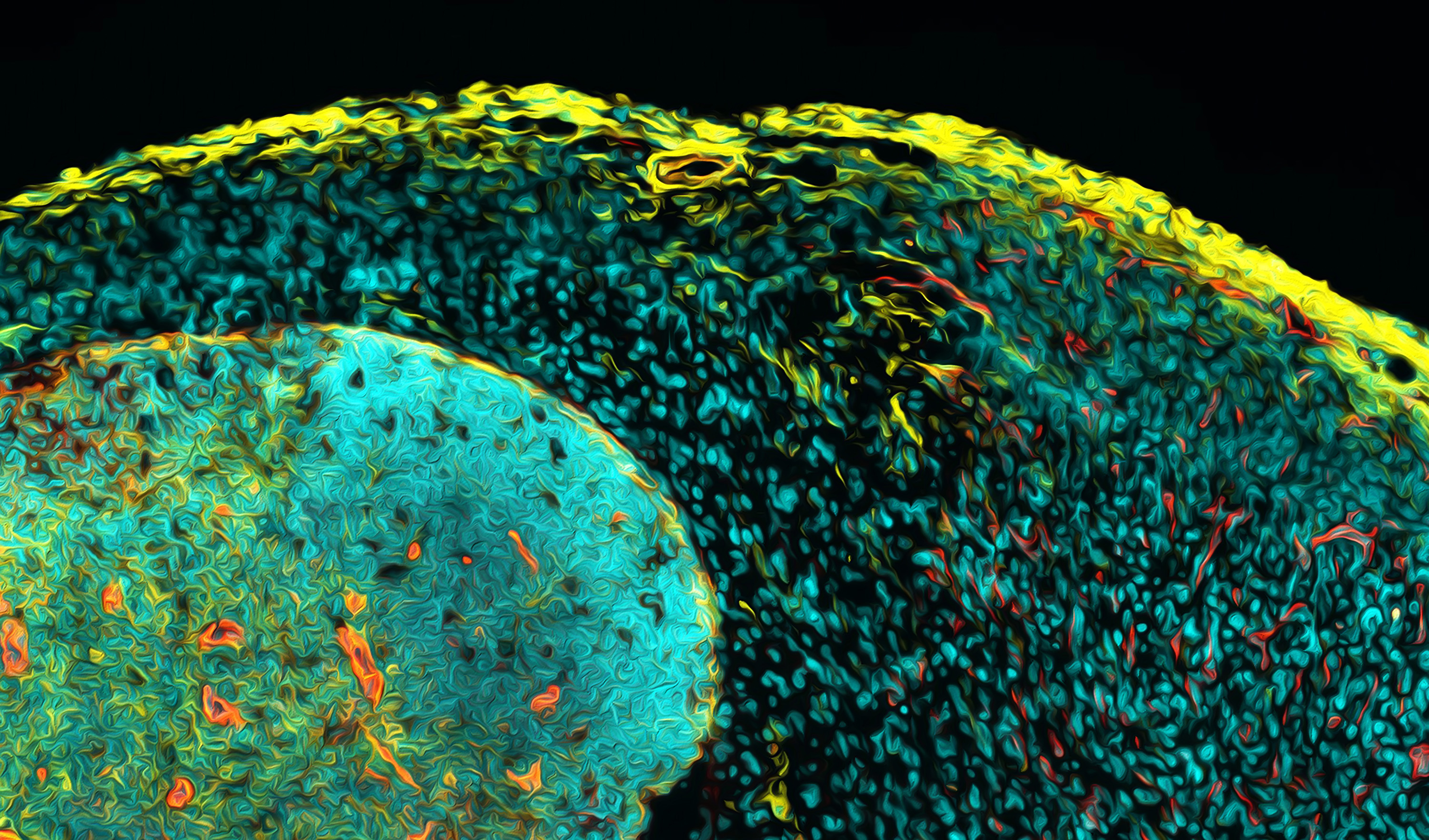
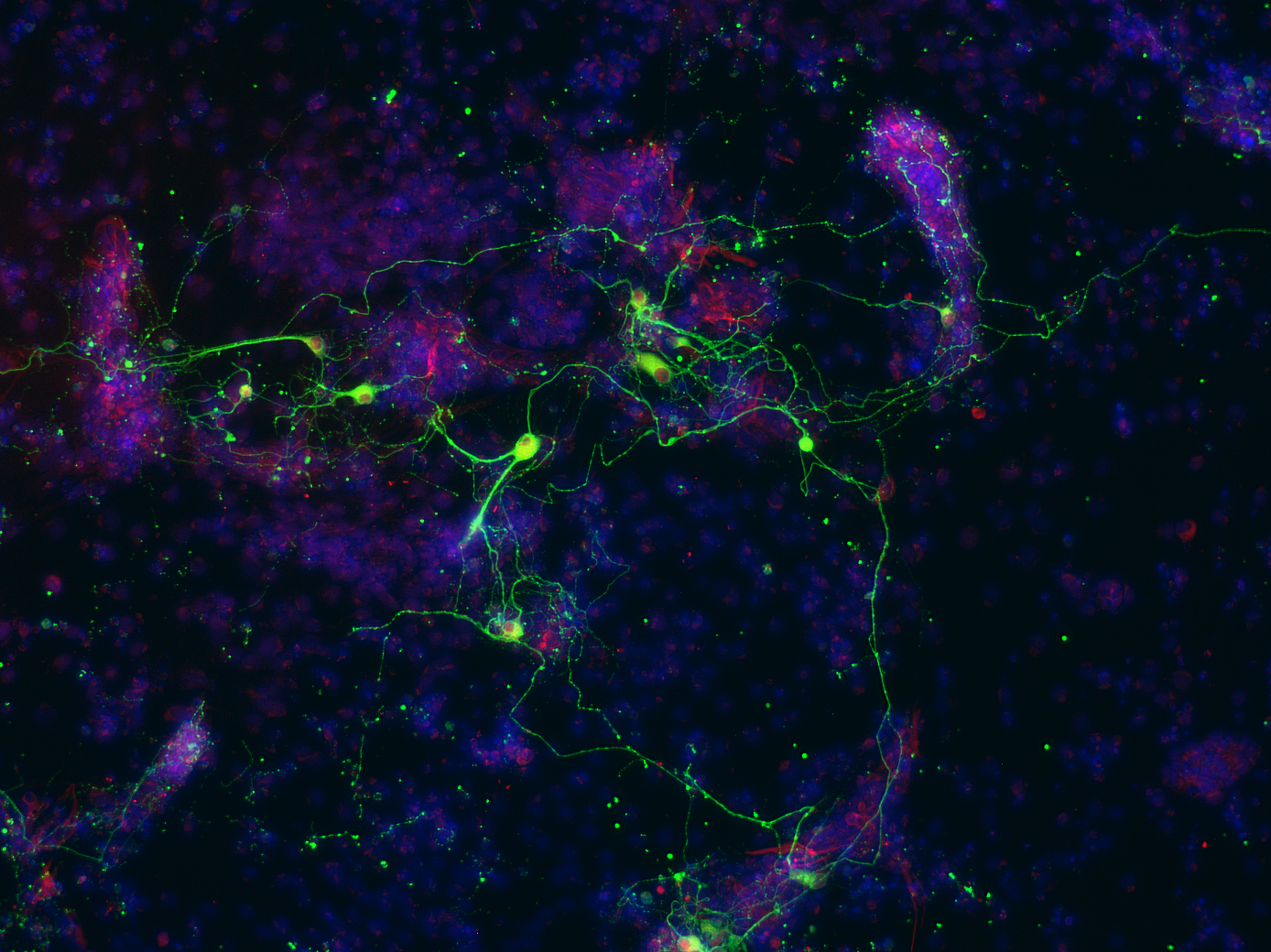
Hematopoietic stem cells (HSCs) are located in the bone marrow and are responsible for maintaining the lifelong production of a series of progenitors and all mature blood cells. HSCs are rare, multipotent and display lifelong self-renewal activity. Research at HI-STEM includes studies on the molecular and cellular basis of HSC self-renewal, HSC dormancy, HSC ageing, HSC epigenome and enhancers, HSCs and progenitors at a single cell level, HSCs and inflammation, HSC niches and genomic stability of HSCs.
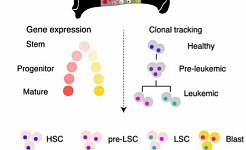
Leukemia is a group of blood cancers that usually initiate in the bone marrow and result in high numbers of abnormal white blood cells. Acute myeloid leukemias is an aggressive form of leukemia which evolves from HSCs that carry a single initiating mutation. These pre-leukemic cells form a clone and subsequent mutations within cells of this clone lead to the development of full blown AML, which is treated by a standardized chemotherapy regime. Unfortunately, patients frequently relapse due to therapy resistant leukemic stem cells. Research at HI-STEM includes studies on leukemia initiating epigenetic events, molecular basis of leukemic stem cells and therapy resistance, leukemia at the single cell level, characterization of minimal-residual–disease and development of novel treatment strategies.
HI-STEM Groups active in this topic: Marieke Essers, Simon Haas, Daniel Hübschmann (bioinformatics analysis), Michael Milsom. Andreas Trumpp
Highlight Publications:
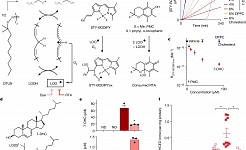
7-Dehydrocholesterol is an endogenous suppressor of ferroptosis (Nature, 2024)
Combinatorial BCL2 Family Expression in Acute Myeloid Leukemia Stem Cells Predicts Clinical Response to Azacitidine/Venetoclax (Cancer Discovery, 2023)
Identification of leukemic and pre-leukemic stem cells by clonal tracking from single-cell transcriptomics (Nature Communications, 2021)
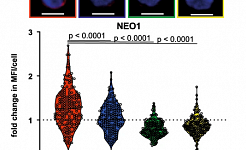
Niche derived netrin-1 regulates hematopoietic stem cell dormancy via its receptor neogenin-1 (Nature Communications, 2021)
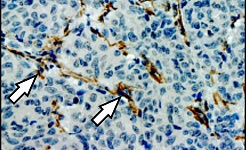
II. Solid Tumors, Metastasis and Therapy Resistance
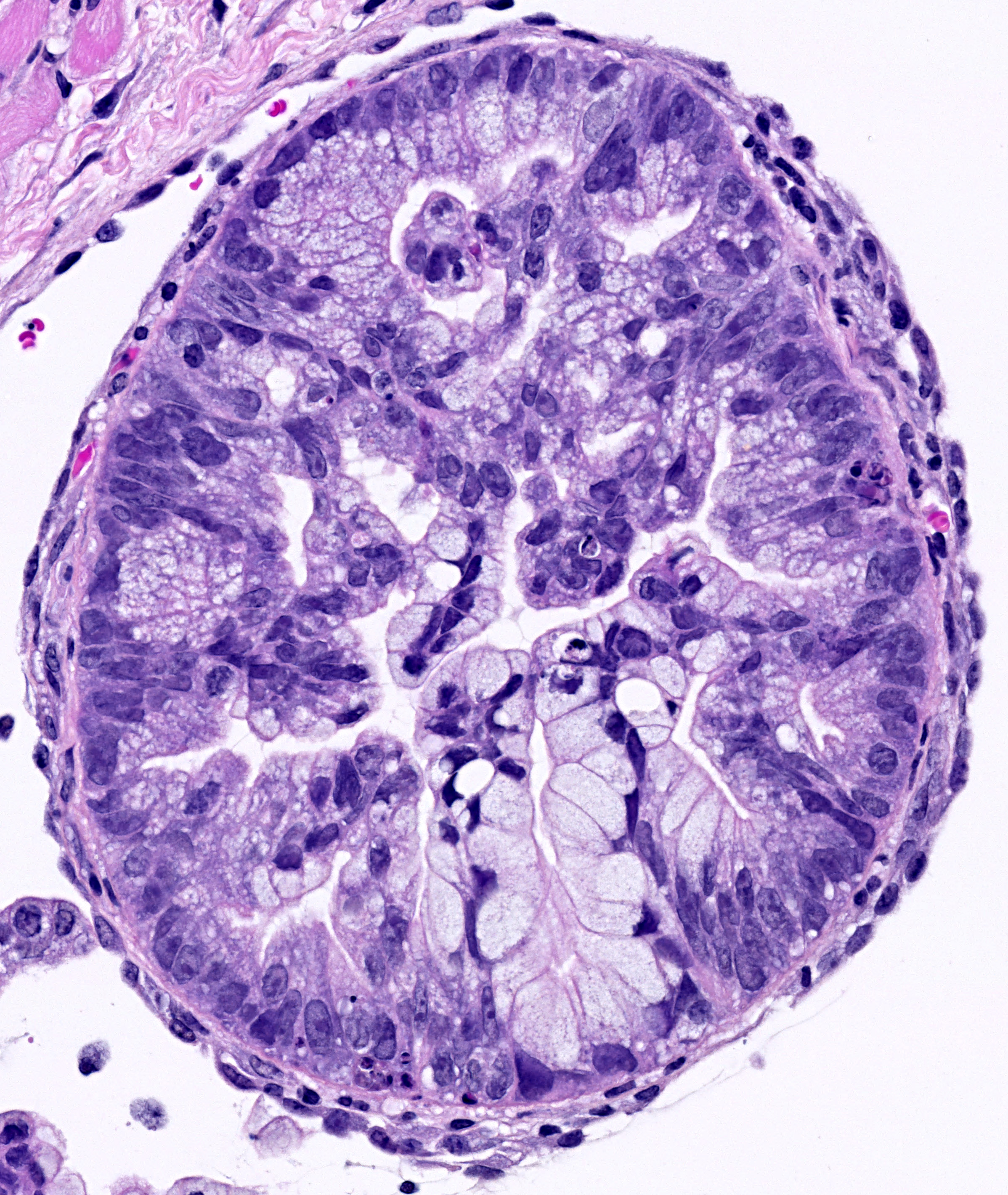
Solid cancers are complex and dynamic three-dimensional structures. They are comprised of genetically altered tumor cells intermingled within a specific tumor microenvironment. Genetic alterations of stem cells or progenitors lead to the generation of “cancer stem cells” (CSCs) that drive tumorigenesis and metastasis. Starting with patient derived mouse models, we extend our work to the analysis of primary patient samples (tumor- and metastasis samples, blood, bone marrow) and link the results to clinical parameters with the goal to develop innovative strategies to detect and target cancer and metastasis cells including CSCs and break therapy resistance.
Research at HI-STEM studies carcinomas of the mammary gland, pancreas, ovary and kidney and include mechanistic understanding and break of therapy resistance, circulating tumor cells and liquid biopsy, epigenomic repgrogramming, stemness and de-differentiation, cancer cells at the single cell level.
HI-STEM Groups active in this topic: Daniel Hübschmann (bioinformatics analysis), Thordur Oskarsson, Martin Sprick, Andreas Trumpp
Highlight Publications:
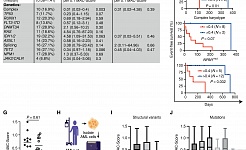
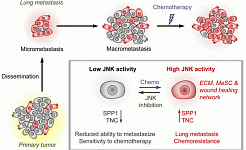
Metastasis-initiating cells induce and exploit a fibroblast niche to fuel malignant colonization of the lungs (Nature Communications, 2020)
Stress signaling in breast cancer cells induces matrix components that promote chemoresistant metastasis (EMBO Molecular Medicine, 2018)
CYP3A5 mediates basal and acquired therapy resistance in different subtypes of pancreatic ductal adenocarcinoma (Nature Medicine, 2016)
Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay (Nature Biotechnology, 2013)
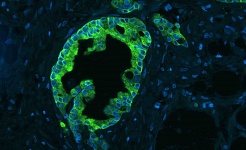
III. Stem Cells and Tumor Microenvironment
Solid tumors are comprised of genetically altered cancer cells intermingled within a specific tumor microenvironment (stroma) that consists of genetically normal cells that have adapted towards malignant growth. This tumor microenvironment includes surrounding blood vessels, fibroblasts, bone marrow-derived inflammatory cells, lymphocytes and other immune cells, which are interconnected by a rich extracellular matrix that also contains embedded signaling molecules. This organization is similar to stem cell niches in many aspects and thus, the tumor stroma plays the role of a corrupt stem cell niche driven by malignant cells.
Research at HI-STEM includes the development of metastatic niches, cell-cell and cell-matrix interactions and signaling, consequences of chemotherapy on niches in breast cancer and in leukemias, bone marrow niches and inflammatory niches, reactive stroma in pancreatic cancer and niches at the single cell level.
HI-STEM Groups active in this topic: Marieke Essers, Simon Haas, Thordur Oskarsson, Martin Sprick, Andreas Trumpp